Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Yasuda, Kenichiro; Sakurai, Satoshi; Gunji, Hideho; Usuda, Shigekazu
Journal of Nuclear Science and Technology, 39(Suppl.3), p.552 - 555, 2002/11
In order to contribute to the strengthened safeguards system based on the Program 93+2 of the IAEA, Japan Atomic Energy Research Institute (JAERI) constructed the CLEAR facility (Clean Laboratory for Environmental Analysis and Research) and is developing analytical technology for ultra trace amounts of nuclear materials in environmental samples. To avoid cross-contamination among the samples and contamination of the clean rooms, radioactive materials in the samples to be introduced into the CLEAR facility will be limited to a certain amount. For this purpose the authors have examined the feasibility of Imaging-plate method, which is a kind of autoradiography and is suitable for determination on distribution of low-level radioactivity in the samples. Preliminary examination with  -ray (K-40), the linearity was obtained in the range of 0.01 - 0.2 Bq. The experiments with
-ray (K-40), the linearity was obtained in the range of 0.01 - 0.2 Bq. The experiments with  -ray (Sm-147) suggested the detection limit of 0.01 Bq, which was equivalent to 2
-ray (Sm-147) suggested the detection limit of 0.01 Bq, which was equivalent to 2  g of natural uranium. At the presentation, the results on actual environmental samples will be reported.
g of natural uranium. At the presentation, the results on actual environmental samples will be reported.
Iwamoto, Akira
Suri Kagaku, (465), p.10 - 17, 2002/03
no abstracts in English
Kato, Hiroshige*; Mine, Tatsuya*; Mihara, Morihiro; Oi, Takao; Honda, Akira
JNC TN8400 2001-029, 63 Pages, 2002/01
Cementitious materials will be used for the TRU waste repository as a component of engineered barrier system. The distribution coefficients which represent the retardation of radionuclides migration for the cementitious materials would be one of the important parameter for the safety assessment. The much information of radionuclide sorption onto the cementitious materials has been accumulated through the study in the world. Therefore it is necessary to compile the information and Kd of the radionuclides reported in previous studies. In this report, the Kd of the important radionuclides, such as C, Ni, Se, Sr, Zr, Nb, Mo, Tc, Sn, I, Cs, Sm, Pb, Ra, Ac, Th, Pa, U, Np, Pu, Am, Cm, for the cementitious materials were compiled as the Sorption Database (SDB). For radionuclides to be sensitive to the redox potential, e.g. Se, Tc, Pa, U, Pu and Np, some Kds measured under the controlled atmosphere had been reported, and few Kds measured under the controlled redox potential had been reported. For Se, Mo, Sm, Cm and Ac, the distribution coefficients had not been reported, therefore distribution coefficients of Se and Mo for OPC (Ordinary Portland Cement) pastes were measured by batch sorption experiments and these data were added into the SDB.
Kitamura, Akira; *
JNC TN8400 2001-009, 54 Pages, 2001/01
Spectroscopic measurements of neodymium(III) and samarium(III) were carried out by spectrophotometer and laser-induced photoacoustic spectroscopic (LPAS) system for the investigation of the detection limit of both systems. The absorption spectra and photoacoustic spectra of Nd and Sm
and Sm were obtained with varying the concentration of the ions from 2
were obtained with varying the concentration of the ions from 2 10
10 to 2
to 2 10
10 mol
mol dm
dm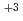 . The absorption spectrum of Nd
. The absorption spectrum of Nd was also determined by a special spectrophotometer, of which the measurement cell was set in a glove box filled with inert nitrogen gas. For the comparison with these photoacoustic and absorption spectra, the absorption spectra of Nd
was also determined by a special spectrophotometer, of which the measurement cell was set in a glove box filled with inert nitrogen gas. For the comparison with these photoacoustic and absorption spectra, the absorption spectra of Nd and Sm
and Sm were determined by an usual spectrophotometer with the light-path lengths of 1 cm and 10 cm. The detection limit of the photoacoustic measurement was reported much lower than that of absorbance measurement by several researchers. However, the present study was concluded that the detection limit of photoacoustic measurement with the present LPAS system was similar to that of absorbance measurement with the light-path length of 10 cm. The detection limits of neptunium(IV,V) were estimated and the possibility of the speciation of neptunium(IV) was discussed from the results of the present study.
were determined by an usual spectrophotometer with the light-path lengths of 1 cm and 10 cm. The detection limit of the photoacoustic measurement was reported much lower than that of absorbance measurement by several researchers. However, the present study was concluded that the detection limit of photoacoustic measurement with the present LPAS system was similar to that of absorbance measurement with the light-path length of 10 cm. The detection limits of neptunium(IV,V) were estimated and the possibility of the speciation of neptunium(IV) was discussed from the results of the present study.
Fujii, Yasunori*; Iwamoto, Akira; Fukahori, Tokio; Onuki, Toshihiko; Nakakawa, Masayuki; Hidaka, Hiroshi*; Oura, Yasutsugu*; M ller, P.*
ller, P.*
Nuclear Physics B, 573(1-2), p.377 - 401, 2000/05
Times Cited Count:191 Percentile:97.63(Physics, Particles & Fields)no abstracts in English
; ; Ishiguro, Katsuhiko; Nakajima, Kunihiko*;
JNC TN8400 99-087, 41 Pages, 1999/11
Corrosion of the carbon steel overpack leads to a volume expansion since the specific gravity of corrosion products is smaller than carbon steel. The buffer material is compressed due to the corrosive swelling, reducing its thickness and porosity. On the other hand, Buffer material may be extruded into fractures of the surrounding rock and this may lead to a deterioration of the planned functions of the buffer, including retardation of nuclides migration and colloid filtration. In this study, the sensitivity analyses for the effect of volume expansion and intrusion of the buffer material on nuclide migration in the engineering barrier system are carried out. The sensitivity analyses were performed on the decrease in the thickness of the buffer material in the radial direction caused by the corrosive swelling, and the change in the porosity and dry density of the buffer caused by both compaction due to corrosive swelling and intrusion of buffer material. As results, it was found the maximum release rates of relatively shorter half-life nuclides from the outside of the buffer material decreased for taking into account of a volume expansion due to overpack corrosion. On the other hand, the maximum release rates increased when the intrusion of buffer material was also taking into account. It was, however, the maximum release rates of longer half-life nuclides, such as Cs-137 and Np-237, were insensitive to the change of buffer material thickness, and porosity and dry density of buffer.
Sato, Haruo
JNC TN8400 99-062, 16 Pages, 1999/10
Effective diffusion coefficients (De) for Ni , Sm
, Sm , Am
, Am and SeO
and SeO were measured as a function of the ionic charge of diffusion species to quantitatively evaluate the effect of ionic charge in compacted bentonite. The De measurements for Ni
were measured as a function of the ionic charge of diffusion species to quantitatively evaluate the effect of ionic charge in compacted bentonite. The De measurements for Ni and Sm
and Sm were carried out for a bentonite dry density of 1.8 Mg
were carried out for a bentonite dry density of 1.8 Mg m
m with a simulated porewater condition of pH5
with a simulated porewater condition of pH5 6 by through-diffusion method. The De values for SeO
6 by through-diffusion method. The De values for SeO were measured for a bentonite dry density of 1.8 Mg
were measured for a bentonite dry density of 1.8 Mg m
m with a simulated porewater condition of pH11. The De measurements for Am
with a simulated porewater condition of pH11. The De measurements for Am were carried out for the dry densities of 0.8, 1.4 and l.8 Mg
were carried out for the dry densities of 0.8, 1.4 and l.8 Mg m
m with a porewater condition of pH2 in order to check cation exclusion. Sodium bentonite, Kunigel-V1 was used for those measurements. For the measurements of Am, H-typed Kunigel-V1 which interlayer ion (Na
with a porewater condition of pH2 in order to check cation exclusion. Sodium bentonite, Kunigel-V1 was used for those measurements. For the measurements of Am, H-typed Kunigel-V1 which interlayer ion (Na ) was exchanged with H
) was exchanged with H was used, because the experiments are carried out for a low pH range. The order of obtained De values was Sm
was used, because the experiments are carried out for a low pH range. The order of obtained De values was Sm
 Ni
Ni
 Am
Am
 SeO
SeO . These De values were compared to those reported to date. Consequently, the order of De values was Cs
. These De values were compared to those reported to date. Consequently, the order of De values was Cs
 Sm
Sm
 HTO
HTO  Ni
Ni
 anions (I
anions (I , Cl
, Cl , CO
, CO , SeO
, SeO TcO
TcO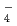 , NpO
, NpO CO
CO , UO
, UO (CO
(CO )
)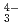 ), showing a tendency of cations
), showing a tendency of cations  HTO
HTO  anions. Only the De values of Am
anions. Only the De values of Am were approximately the same degree as those of anions. The reason that the De of Ni
were approximately the same degree as those of anions. The reason that the De of Ni was lower than that of HTO may be because the free water diffusion coefficient (Do) of Ni
was lower than that of HTO may be because the free water diffusion coefficient (Do) of Ni is about 1/3 of that of HTO. The cause that the De of Am
is about 1/3 of that of HTO. The cause that the De of Am was approximately the same degee as those of anions may be because the Do of Am
was approximately the same degee as those of anions may be because the Do of Am is about 1/3 of that of HTO and that Am
is about 1/3 of that of HTO and that Am was electrostatically repulsed from the surface of bentonite by cation exclusion. The formation factors (FF), calculated normalizing Do, were in the ...
was electrostatically repulsed from the surface of bentonite by cation exclusion. The formation factors (FF), calculated normalizing Do, were in the ...
*; Sato, Haruo; *
JNC TN8400 99-059, 59 Pages, 1999/10
Organic acids in groundwater are considered to form complexes and increase the solubility of radionuclides released from vitrified waste in a high-level radioactive waste (HLW) repository. To ivestigate whether the solubility of samarium (Sm) is influenced by organic substances, we measured Sm solubility in the presence of different organic substances and compared those values with results from thermodynamic predictions. Humic acid (Aldrich) is commercially available and soluble organic matter originated from bentonite were used as organic substances in this study. Consequently, the solubility of Sm showed a tendency to apparently increase with icreasing the concentration of humic acid, but in the presence of carbonate, thermodynamic predictions suggested that the dominant species are carbonate complexes and that the effect of organic substances are less than that of carbonate. Based on total organic carbon (TOC), the increase of Sm solubility measured with humic acid (Aldrich) was more significant than that in the case with soluble organic matter originated from bentonite. Since bentonite is presumed to include also simple organic matters of which stability constant for forming complexes is low, the effect of soluble organic matter originated from bentonite on the solubility of Sm is eonsidered to be less effective than that of humic acid (Aldrieh). Experimental values were compared with model prediction, propsed by Kim, based on data measured in a low pH region. Tentatively we calculated the increase in Sm solubility assuming complexation with humic acid. Trial calculations were carried out on the premise that the complexation reaction of metal ion with humic acid is based on neutralization process by 1-1 complexation. In this process, it was assumed that one metal ion coordinates with one unit of complexation sites which number of proton exchange sites is equal to ionic charge. Consequently, Kim's model indicated that carbonate complexes should be dominant ...
Ito, Takao; Yamazaki, Haruyuki*; Usui, Katsutomi; Mogaki, Kazuhiko; Kuriyama, Masaaki
JAERI-Tech 99-066, p.13 - 0, 1999/09
no abstracts in English
Kimura, Takaumi; Kato, Yoshiharu
Journal of Alloys and Compounds, 275-277, p.806 - 810, 1998/00
Times Cited Count:110 Percentile:96.67(Chemistry, Physical)no abstracts in English
Kimura, Takaumi; Kato, Yoshiharu
Journal of Alloys and Compounds, 278, p.92 - 97, 1998/00
Times Cited Count:69 Percentile:92.91(Chemistry, Physical)no abstracts in English
Sumiya, Shuichi; Hayashi, Naomi; ; Narita, Osamu
PNC TN8430 91-001, 45 Pages, 1990/12
A radioanalytical method for low level samarium-151(Sm-151) and promethium-147(Pm-147) in environmental samples has been studied for the environmental assessment around nuclear facilities. In this study, we use the separation method with high performance liquid chromatography (HPLC) to determine Sm-151 and Pm-147 in environmental samples such as sea sediments and marine organisms. Samarium-151 and Pm-147 in environmental samples are coprecipitated with other lanthanoids after adding neodymium(Nd). These nuclides are purified by anion exchange methods in methanol-mineral acid media. After the purification, Sm-151 and Pm-147 are separated with HPLC in lactic acid-sodium hydroxide media, and determined with liquid scintillation counting, respectively. The Nd is determined by inductively coupled plasma atomic emission spectrometry (ICP-AES) to correct chemical recoveries of these nuclides. The detection limits for Sm-151 and Pm-147 in this method are about 0.01Bq/sample.
*; Shinohara, Yoshikuni
JAERI-M 5712, 36 Pages, 1974/05
no abstracts in English
Shinohara, Yoshikuni; Kitamura, Masaharu*
JAERI-M 5664, 34 Pages, 1974/04
no abstracts in English
*; *; *; ; *; *
Bunseki Kagaku, 13(1), p.32 - 38, 1964/00
no abstracts in English
Sato, Makoto; Ichimura, Seiji; Suyama, Kenya; Tonoike, Kotaro; Kamohara, Keiko*; Suzuki, Norihisa*
no journal, ,
no abstracts in English